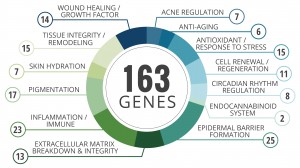
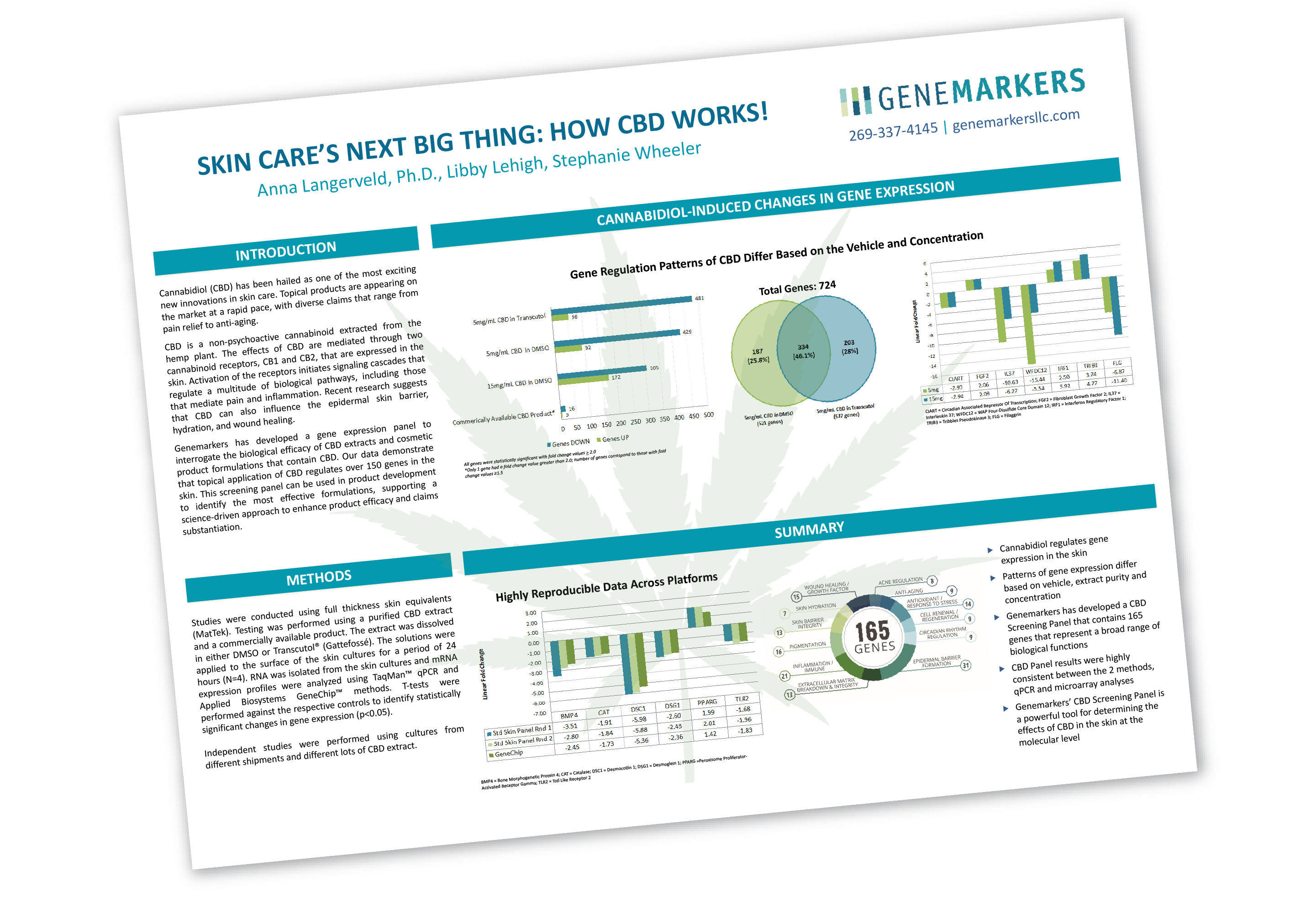
Genemarkers Receives Pure Michigan Business Connect Grant to Begin COVID-19 Testing
Genemarkers is honored to be one of 12 small businesses and nonprofits selected to receive the Pure Michigan Business Connect COVID-19 Emergency Access and Retooling Grants program for $104K to provide COVID-19 (SARS-CoV-2) testing in their CLIA-certified laboratory in downtown Kalamazoo, Michigan.
Genemarkers began hearing the call from medical and public health experts across the country for more COVID-19 testing earlier this winter. As experts in high complexity testing, Genemarkers’ scientists have been actively planning for the addition of this test to the Company’s existing genomic testing services.
Genemarkers has over 10 years of experience with Thermo Fisher qPCR assays and will leverage this experience to offer the FDA’s Emergency Use Authorization (EUA) TaqPath™ COVID-19 Combo Kit. The test is designed to detect the virus that causes COVID-19 in respiratory specimens for nasal or oral swabs. The Company will validate the test as a Laboratory Developed Test (LDT) so it can perform testing in a 384-well format to provide optimal testing capacity and turnaround time. “We are grateful for the opportunity to join the fight against this disease while doing what we do best,” states Dr. Anna Langerveld, President and Chief Scientific Officer.
Genemarkers has a long history of stepping up to the plate when it comes to public health crises, most recently genetic research related to opioid addiction. “Genemarkers is proud to begin providing this crucial service to address the COVID-19 pandemic,” states Mike Getz, Chief Executive Officer.